Neurotrophin mimetics: recent frontiers in neurodegenerative disorders
|
One of the main causes of neurodegeneration relies in changes of the expression of neurotrophins (NTs) and/or their receptors. Indeed, imbalances between NTs and their receptors (TrkA, TrkB, TrkC and p75NTR) or changes in their activity, lead to neuronal damage resulting in neurological and neurodegenerative conditions. The therapeutic role of neurotrophins attracted the attention of many scientists during the years but their poor pharmacokinetic properties, such as reduced bioavailability, inability to penetrate the BBB and short half-life make the large neurotrophin proteins not suitable as drugs (Josephy-Hernandez et al. 2017).
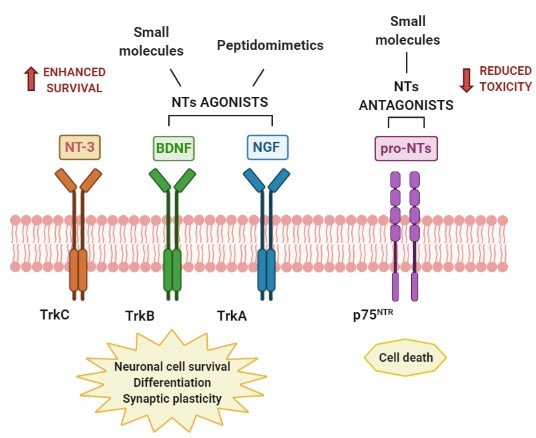
For this reason, several efforts are ongoing for the development of neurotrophins mimetics (small molecules and peptidomimetics) that can modulate the action of Trks and/or p75NTR receptors with improved pharmacodynamic and pharmacokinetic properties. Specifically, the neurotrophin mimetics can be classified in TrkA and TrkB receptor agonists and, on the other hand, in p75NTR antagonists, as summarized in Figure 1 (Josephy-Hernandez et al. 2017; Longo et al. 2013). TrkA AGONISTS
Among the TrkA agonists, the small molecule gambogic amide exerts a potent neurotrophic activity decreasing apoptosis in primary hippocampal neurons (Jang et al. 2007); the non-peptidic agonist of TrkA, MT2, protects neurons from Aβ amyloid-mediated death in NGF-deficient neurons (Scarpi et al. 2012) and Talaumidin and its derivatives show neuroprotective effects, promoting neurite outgrowth in PC12 cells, selective for TrkA receptor (Harada et al. 2020). More impressive is the peptidomimetic Cerebrolysin, known for its protective role in Alzheimer’s disease (AD) (Alvarez et al. 2006). Indeed, in a double-blind trial, it was able to improve the daily activities and psychiatric symptoms in patients with mild to severe AD, after intravenous administration (Alvarez et al. 2011). In addition, the cyclic peptide Tavilermide (MIM-D3), acting as a selective TrkA agonist, showed a relevant improvement of cognitive capacities of treated aged rats, leading to selective survival of the cholinergic neurons (Bruno et al. 2004). Currently, it is in Phase 3 clinical trials for treating the signs and symptoms of dry eye (NCT03925727). TrkB AGONISTS A large number of TrkB agonists mimetics have been studied for neurodegeneration. Among these, some deserve more consideration; deoxygedunin, with a selective TrkB activity, is able to promote axon regeneration in topical treatments (English et al. 2013) and it is proven to be efficient in two Parkin-son’s disease (PD) animal models, leading to the protection of locomotor function and the reduction of neuronal death in dopaminergic neurons (Nie et al. 2015). A number of studies corroborated that the flavonoid 7,8-dyhydroxyflavone (7,8-DHF) shows neuroprotection in PD and Huntington's disease (HD) models (Jang et al. 2010) (Jiang et al. 2013) together with antioxidant activity (Chen et al. 2011) and enhances motor neuron survival, motor function and spine density in an amyotrophic lateral sclerosis (ALS) model (Korkmaz et al. 2014). The benzothiazole riluzole has neuroprotective effect and it has been approved in the United States in 1995 for the treatment of ALS, increasing BDNF and GDNF levels with improvement of motor neuron survival (Dennys et al. 2015). It is also under several clinical trials in combination with other drugs. Brimonidine exerts neuroprotective effect in retinal ganglion cells (RGCs) through up-regulation of the expression of BDNF in these cells (Gao et al. 2002). Brimonidine is used in the treatment of glaucoma as eye drops to reduce intraocular pressure (IOP) under the brand name Lumify®. Different drugs, used against PD such as Rotigotine, Selegiline, Rasagiline, Memantine and Levodopa interact with TrkB and increase BDNF expression. Furthermore, of particular note, Massa et al. discovered small molecules neurotrophic mimetics with specificity for TrkB at nM concentration (Massa et al. 2010). One of these, LM22A-4, prevents neuronal death in in vitro models of AD, HD and PD (Longo et al. 2013). p75NTR ANTAGONISTS In this class it is worthwhile to highlight the small non peptide LM11A-31 developed by Massa et al (Simmons et al. 2014). Oral administration in AD mice models reduces degeneration of cholinergic neurites (Simmons et al. 2014). Furthermore, with a direct activation of p75NTR signalling and inhibition of the apoptotic pathway improves motor function in spinal cord injury (SCI) mouse model and leads to an antiapoptotic effect in mice after traumatic brain injury (TBI) (Tep et al. 2013) (Shi et al. 2013). Neurosteroids as potent neurotrophin mimetics Neurosteroids affect survival, development and function of neurons and it has been found that their levels in the brain reduce in neurodegenerative conditions. Recent studies have shown that the neurosteroid dehydroepiandrosterone (DHEA) acts as a neurotrophic factor in the brain and prevents neu-ronal apoptosis by interacting with the neurotrophin receptors TrkA and p75NTR (Lazaridis et al. 2011). In addition, DHEA regu-lates microglia inflammation via TrkA dependent signalling and reduces the inflammation, downregulating the expression of the pro-inflammatory cytokines (Alexaki et al. 2018). Neverthe-less, DHEA is metabolized in humans into estrogens and andro-gens, thus, its long-term administration increases the risk for hormone-dependent cancer. Therefore, DHEA analogues with modifications at position C17 of the steroid skeleton were synthesized aiming to improve the antiapoptotic activity and neuroprotection of the parent molecule, without the undesired hormonal side effects. The C17-spiroepoxy DHEA analogues BNN27 and BNN20 are remarkable. These steroid derivatives, synthesized by Calogeropoulou et al. (Calogeropoulou et al. 2009) are able to cross the BBB without the undesired hormonal side effects of DHEA and they are selective for TrKA and TrkB, respectively. Specifically, BNN27 activates TrkA, ex-erting antiapoptotic effect in PC12 cells and it increases survival of mouse motor neurons co-cultured with human astrocytes from SOD1 ALS patients. In vivo studies showed an antiapoptotic effect and reduction of the toxic effect of cuprizone in oligodendrocytes restoring the myelin loss (Pediaditakis et al. 2016) (Bonetto et al. 2017) (Glajch et al. 2016). In addition, BNN27 interacts with p75NTR inhibiting apoptosis in primary cultures of cerebellar granule neurons (CGNs), specific for p75NTR (Pediaditakis et al. BNN27, 2016). On the other hand, BNN20 binds with high affinity to TrkB, showing antiapoptotic activity in vitro. Its neuroprotective activity was analysed in Weaver mouse genetic model of PD where long term administration of BNN20 protects dopaminergic neurons by mimiking BDNF and induces antiapoptotic, antioxidant and anti-inflammatory effects (Botsakis et al. 2017). Conclusion All these results point out that several small molecules possess promising activity against neurodegenerative disorders and lay the foundation for the future development of new neurotrophin mimetics with BBB-permeability and selective neuroprotective and neurogenic activities with potential application in neurodegenerative diseases. |
Alessia joined the EuroNeurotrophin network as ESR2 at the National Hellenic Research Foundation, Athens, Greece. Her work involves the synthesis of chiral 17-spiro DHEA derivatives, which will be further elaborated to introduce pharmacophore groups, using asymmetric organocatalysis, biomimetic approaches and other synthetic methodologies. Her project also focuses to analyse SNAP PK data on lead compounds and to label steroidal neurotrophin mimetics with fluorophores or NIR-dyes.
|